how many valence electrons are in as|how many valence electrons does mg have : Manila In chemistry, the valence (US spelling) or valency (British spelling) of an atom is a measure of its combining capacity with other atoms when it forms chemical compounds or .
WEB8 de mar. de 2023 · FC Bayern München is going head to head with Paris Saint-Germain starting on 8 Mar 2023 at 20:00 UTC at Allianz Arena stadium, Munich city, Germany. .
0 · valence electrons for each group
1 · how to find valence electrons in copper
2 · how to count valence electrons
3 · how to calculate valence
4 · how many valence electrons does phosphorus have
5 · how many valence electrons does mg have
6 · how many valence electrons does li have
7 · how many valence electrons does cl have
8 · More
webConsultório Médico – Angelica II. Av. Angélica , 2229 - Santa Cecília. São Paulo - SP, 01228-904
how many valence electrons are in as*******sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 .The electrons that determine valence – how an atom reacts chemically – are those with the highest energy. For a main-group element, the valence electrons are defined as those electrons residing in the electronic shell of highest principal quantum number n. Thus, the number of valence electrons that it may have depends on the electron configuration in a simple way. For example, the electronic c.
However, as the previously filled 4th shell (4s) has 2 electrons and is apparently the outermost shell, the number of .
Valence electrons are involved in the formation of chemical bonds between atoms, so it is helpful for chemists to know how many valence electrons each type of atom has. To .how many valence electrons does mg haveSo how many electrons are in the third energy level? Well, there's two and five, for a total of seven. So chlorine has seven valence electrons. And once again, that's very .In chemistry, the valence (US spelling) or valency (British spelling) of an atom is a measure of its combining capacity with other atoms when it forms chemical compounds or .how many valence electrons are in asBased on their position on the periodic table, how many core and valence electrons do the following elements have?(i) Arsenic(ii) Antimony(iii) Strontium(iv) BerylliumCount the valence electrons of the central atom. Add an electron for each bonding atom. Subtract an electron if the central atom has a positive charge; and add an electron for a .The valence band is the band of electron orbitals that electrons can jump out of, moving into the conduction band when excited. The valence band is simply the outermost electron orbital of an atom of any specific .VIDEO ANSWER: To figure out the number of electrons. It's helpful to know the relationship between the number of valence electrons of the main group elements and .
The number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms. This outermost shell is known as the valence shell, and the electrons found in it are called valence electrons. In general, atoms are most stable, least reactive, when their outermost electron shell . You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups (columns) of the periodic table. While these are the most common valences, the real behavior of electrons is less simple.
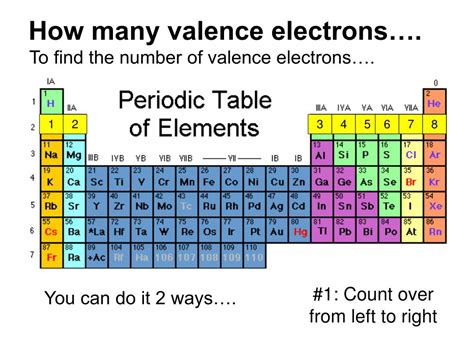
So that means that sodium has one valence electron. And that's very convenient, because sodium is found in group one. And so we can say that for main groups, if you want to figure out . 3.1: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons ..
Arsenic has 5 valence electrons. It's electron configuration is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^3. It's outermost shell (4s and 4p) has 5 electrons, these are the valence electrons. Examples. Magnesium’s ground state electron configuration is 1s 2 2s 2 p 6 3s 2, the valence electrons would be the 3s electrons because 3 is the highest principal quantum number.Magnesium has two valence electrons. Carbon’s ground state electron configuration is 1s 2 2s 2 2p 2.The highest principal quantum number is 2.

2. Find the electron configuration for the element you are examining. Once you know an element's electron configuration, finding its number of valence electrons is quite simple (except, of course, for the transition metals.) If you're given the configuration from the get-go, you can skip to the next step.
About. Transcript. The s-, p-, and d-block elements of the periodic table are arranged into 18 numbered columns, or groups. The elements in each group have the same number of valence .The valence (or valency) of an element is a measure of its combining power with other atoms when it forms chemical compounds or molecules. The concept of valence was developed in the last half of the 19th century and was successful in explaining the molecular structure of many organic compounds. The quest for the underlying causes of valence .The valence electrons are the electrons that determine the most typical bonding patterns for an element. These electrons are found in the s and p orbitals of the highest energy level for the element. Sodium 1s22s22p63s1. Sodium has 1 valence electron from the 3s orbital. Phosphorus 1s22s22p63s23p3. Phosphorus has 5 valence electrons 2 from the . Solution. Steps for Writing Lewis Structures. Example 15.4.1 15.4. 1. 1. Determine the total number of valence electrons in the molecule or ion. Each H atom (group 1) has 1 valence electron, and the O atom (group 16) has 6 valence electrons, for a total of 8 valence electrons. 2.H+ is just a proton, so no electrons would be present. In covalent bonding, the electrons that form the covalent bond do not have to come from each atom. There are instances when an atom donates both electrons to form the covalent bond. A good example of this is when :NH3 reacts with H+. The : represent the lone pair on the nitrogen in :NH3.
You are invited to the channel Martina OLVR🔞🔥.Click above to join.
how many valence electrons are in as|how many valence electrons does mg have